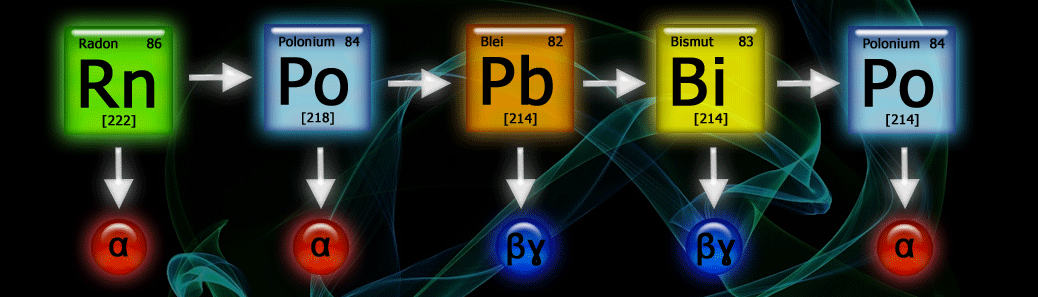
Brief Description of the Processes That Occur After Radon Enters Indoor Air
I will focus here on the essentials. The description should remain generally understandable and provide only the necessary context for comprehending the quantity to be measured and evaluating the measurement results. For more information, I refer you again to various websites..
Radon escapes from the building materials of the room walls into the indoor air (also referred to as radon exhalation) or comes from other parts of the house (such as the basement).
Once released, Rn-222 distributes almost uniformly in the room.
During radioactive decay, the following nuclides are produced in sequence:
- Po-218
- Pb-214
- Bi-214 and
- Po-214.
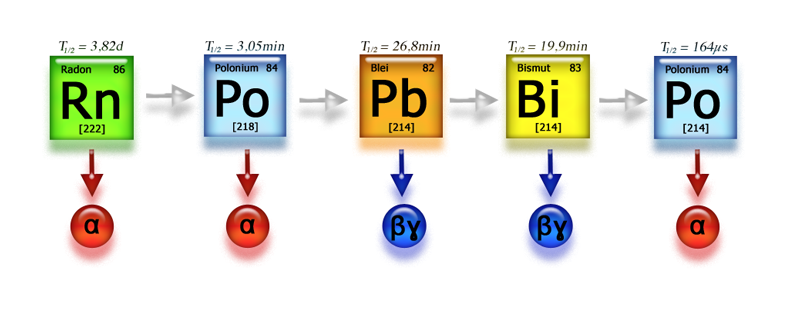
These are referred to as short-lived radon decay products due to their short half-life. After the decay of 214Po, the nuclide 210Pb is formed with a half-life of 22.3 years. Pb-210 and the subsequent nuclides 210Bi and 210Po are classified as long-lived radon decay products. After the alpha decay of Po-210, the stable nuclide Pb-206 is eventually produced. However, the long-lived nuclides of the radon decay chain are not relevant for practical radiation protection considerations and are not discussed further here.
Since the short-lived decay products are heavy metals, which are also partially ionized in the air, they undergo various interactions with air components, such as:
- Formation of clusters, which are structures consisting of the heavy metal atom or ion along with water vapor and trace gas molecules, with particle diameters around 1 nm (10^-6 mm).
- Attachment to dust (aerosol) particles in the air.
Both clusters and aerosol particles are deposited on walls or furnishings at different rates. These interactions result in a portion of the decay products being removed from the air. Therefore, the concentration of decay products in the air is always lower than that of the parent nuclide Rn-222.
In the following image, the processes that the decay products undergo in the air are schematically represented for the first link in the decay chain, Po-218.

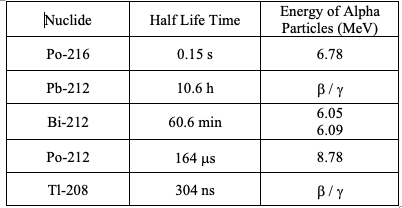
For reference, Table 1 and Table 2 present the half-lives and energies of the alpha radiation of radon and thoron decay products. The values were taken from the “Guideline for the Measurement of Radon, Thoron, and Their Decay Products,” Publications of the German Commission on Radiological Protection – Volume 47.
Table 1: Half-Lives of the Nuclides of Radon Decay Products
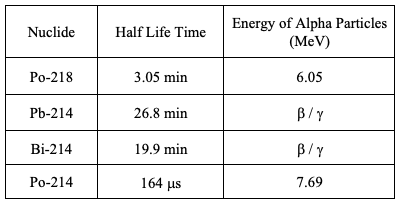
Table 1: Half-Lives of the Nuclides of Thoron Decay Products
(1 MeV ≅ 1.602*10^-4 nJ)
Once again, the key Points in Brief:
- – Radon is always present in indoor air along with its short-lived decay products.
– The activity concentration of radon (gas) is always higher than that of the decay products.
– The radon decay products are responsible for the majority of radiation exposure when inhaled.
– For assessing the “danger” as well as for building a measurement device, it is better to measure the decay products.
At this point, I need to clarify the terms that describe what I actually want to measure – the
Measured quantities:
Activity of a substance is the number of radioactive decays per unit of time. The SI unit is the becquerel. 1 becquerel (1 Bq) corresponds to one decay per second.
Activity concentration (by volume) is the activity of a substance per unit of volume; the unit of measurement is Bq/m³ (becquerel per cubic meter of air).
Potential Alpha Energy Concentration (PAEC) might sound complicated, but it’s not too difficult to understand:
As mentioned earlier, all short-lived decay products are present in the air as a mixture. When inhaled, all of these decay products are retained in the lungs, where they decay, emitting alpha, beta, and gamma radiation. However, for radiation exposure, or more precisely for the dose induced in the lungs, the alpha radiation is almost exclusively relevant. That’s why the definition of PAEC was introduced, as this measurable quantity best characterizes the “danger.”
PAEC is the energy emitted as alpha radiation from a mixture of decay products in a specific volume of air, until their complete decay (down to 210Pb).
To measure the PAEC, a specific volume of air must be drawn through a filter, similar to what happens in the lungs, where the decay products are retained and decay (down to 210Pb). If all the alpha particles emitted from the decay products collected on the filter, from the moment of deposition to complete decay, are detected and counted, the PAEC can be calculated by multiplying the number of alpha particles by their energy and dividing by the volume of air that was drawn through the filter. The energy of the two decay product nuclides that emit alpha particles is 6.0 MeV (Megaelectronvolts) for Po-218 and 7.69 MeV for Po-214.
The unit of measurement is nJ/m³ (nanojoule per cubic meter of air, SI unit); however, the non-SI unit MeV/cm³ is also still commonly used.
Equilibrium Equivalent Radon Concentration (EEC) might sound even more complicated, but it’s quite straightforward. Here’s the definition:
The EEC is the activity concentration of radon that, if in equilibrium with all short-lived decay products, would result in the same PAEC as the mixture being measured. The unit of measurement is Bq/m³, just like the activity concentration (though it refers to the hypothetical activity concentration of radon here).
However, you don’t need to remember everything. PAEC and EEC are the relevant measurements that need to be determined—the goal of the measurement.
It’s important to know that PAEC and EEC have a fixed conversion factor, meaning they can be treated as two different units for the same measurement. The conversion is: 1 Bq/m³ (EEC) corresponds to 5.56 nJ/m³ (PAEC).
I will, of course, describe the calculation of the measurement quantities PAEC and EEC from the measured impulses after the construction instructions, as well as provide some information on the assessment of the measurement values (limit or reference values).